Background: We and others have reported a significant association between low vitamin D levels at diagnosis of indolent B-cell lymphomas and inferior overall survival; the magnitude of this association is stronger than conventional clinical prognostic features. Vitamin D insufficiency impairs rituximab-mediated cytotoxicity and impairs macrophage function, which impacts rituximab responses in vivo. We therefore conducted a phase III, double-bind study of oral cholecalciferol vs. placebo for three years in patients with indolent lymphoma treated with rituximab monotherapy.
Methods: Patients had biopsy-confirmed low tumor burden (GELF criteria) follicular lymphoma (grade 1-3A), marginal zone lymphoma or small lymphocytic lymphoma. Patients with osteoporosis requiring treatment, known symptomatic hyperparathyroidism, hypercalcemia (> normal), history of calcium-related nephrolithiasis, or creatinine > 2X normal were excluded to minimize risk of cholecalciferol therapy. Supplemental vitamin D was discouraged but allowed at doses up to 1000 IU daily. Treatment included rituximab or approved biosimilar 375 mg/m 2 weekly x 4 (or subcutaneous equivalent dosing per investigator discretion). Patients were randomized in a double-blind, 2:1 fashion to receive 2000 IU vitamin D 3 or placebo daily (BioTech Pharmacal Inc) beginning on day 1 of rituximab. Randomization was stratified by histology (follicular vs all other) and FLIPI score (high vs low and intermediate). Restaging PET/CTs were performed at week 13; responding patients continued on study with daily dosing for 36 months or until disease progression. Patients with stable disease or disease progression at week 13 were counted as events. Blinded serum 25(OH) vitamin D2 and D3, was measured by tandem mass spectrometry; saliva was collected for the isolation of germline DNA and SNP analysis using Oragene collection kits, OGR-500 (DNA Genotek Inc). The primary endpoint for this study is the time from randomization to progression or death, right-censored by the time of last follow-up, the cohort included all eligible, treated patients. The study provided 81% power to detect HR = 0.55 at a two-sided α = 0.05 level of significance.
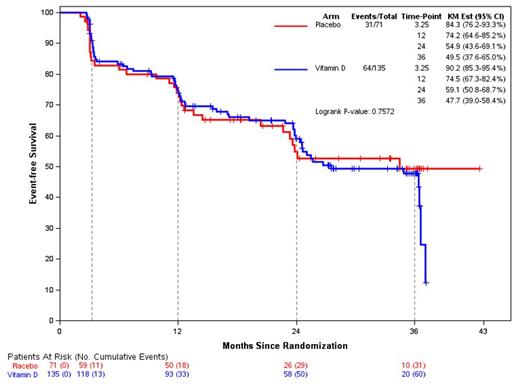
Results: 206 patients (follicular, n=137; MZL, n=33; SLL, n=22 and MALT, n=14) constituted the final analysis cohort. Median age was 62 (range 30-80 years); 57% female; 89% White; and 76% were overweight or obese. The median vitamin D level at baseline was 33ng/mL (range 6-80); at week 13, mean vitamin D level was 41.6 ng/mL in Vit D arm vs. 31.3 ng/mL in the placebo arm; these differences persisted throughout the study confirming compliance. Toxicity was as expected with rituximab; specific events of interest were rare and included hypercalcemia (n=2; all placebo arm); renal calculi (n=4; all vit D arm) and bone fractures (n=3; all vit D arm). At week 13, 172 pts (84%) responded, including CR (n=110) and PR (n=62). There was no difference in response between the arms. Responses by subtype: FL: 93%; SLL: 50%; MZL: 67%; MALT: 83%. With median follow-up of 19.5 months, the median EFS was 34.5 months (95% CI: 24 - 42+ months) for all patients; there was no statistically significant difference in EFS between placebo and vitamin D (p=0.7; Figure 1). Subgroup analysis of histology, FL-IPI score, sex and vitamin D level at enrollment also showed no significant difference in EFS between placebo and vitamin D, including those patients with the lowest tertile of baseline vitamin D level (28 or lower; p=0.27). Polymorphisms of vitamin D receptor binding protein and vitamin D receptor were available in 94% of patients and were balanced between treatment arms; there was no statistically significant association between any specific polymorphism and EFS.
Conclusion: We observed no statistically significant benefit of vitamin D supplementation in patients with indolent lymphoma treated with rituximab in this phase III, placebo-controlled clinical trial. These results suggest that the association of low vitamin D with outcome in this disease likely represents a surrogate for illness and comorbidity rather than a modifiable risk factor, and may have implications for ongoing and planned studies of vitamin D supplementation in other malignancies.
Disclosures
Lossos:Adaptive: Honoraria; NCI: Research Funding; LRF: Membership on an entity's Board of Directors or advisory committees; University of Miami: Current Employment; NCI: Research Funding; BeiGene: Consultancy. Cohen:Lam Therapeutics: Research Funding; BMS/Celgene: Research Funding; BioInvent: Research Funding; Genentech: Research Funding; Novartis: Research Funding; Takeda,: Research Funding; ADCT: Consultancy; AstraZeneca: Consultancy, Research Funding; Abbvie: Consultancy; Janssen: Consultancy; BeiGene: Consultancy; Loxo/Lilly: Consultancy, Research Funding. Reagan:Kite, a Gilead Company: Consultancy, Other: speaker; Seagen: Research Funding; Genentech: Research Funding; Caribou biosciences: Consultancy. Casulo:Abbvie: Consultancy; Follicular Lymphoma Foundation: Other: Leadership role; Lymphoma Research Foundation: Other: Leadership Role; Verastem: Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Gilead Sciences: Research Funding; SecuraBio: Research Funding; GenMab: Research Funding; Genentech: Consultancy, Research Funding. Barr:Seattle Genetics: Consultancy; Celgene: Consultancy; AstraZeneca: Consultancy, Research Funding; Gilead: Consultancy; Janssen: Consultancy; MEI Pharma: Consultancy; Genentech: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy; Morphosys: Consultancy; TG therapeutics: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy; AbbVie: Consultancy. Leonard:National Cancer Institute, Leukemia and Lymphoma Society, Genentech, Epizyme, Janssen: Research Funding; AbbVie, AstraZeneca, Astellas, Bayer, BeiGene, BMS, Calithera, Constellation, Eisai, Epizyme, GenMab, Grail, Incyte, Janssen, Karyopharm, Lilly, Merck, Mustang Bio, Pfizer, Roche/Genentech, Seagen, Second Genome, Sutro: Consultancy. Nastoupil:Genentech, Inc., Genmab, Gilead/Kite, Janssen, Merck, Novartis, Takeda: Honoraria, Research Funding; Gilead Sciences/Kite Pharma: Honoraria, Research Funding; DeNovo: Honoraria; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; Caribou Biosciences: Honoraria, Research Funding; AstraZeneca: Honoraria; ADC Therapeutics: Honoraria; Regeneron: Honoraria; Daiichi Sankyo: Honoraria, Research Funding; AbbVie: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal